39 fda structured product labels
Indexing Structured Product Labeling | FDA This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) will index the content of labeling for human drug... SPL Standard Training | FDA A series of Structured Product Labeling (SPL) training opportunities are being offered to individuals responsible for the preparation and submission of information to be provided in SPL...
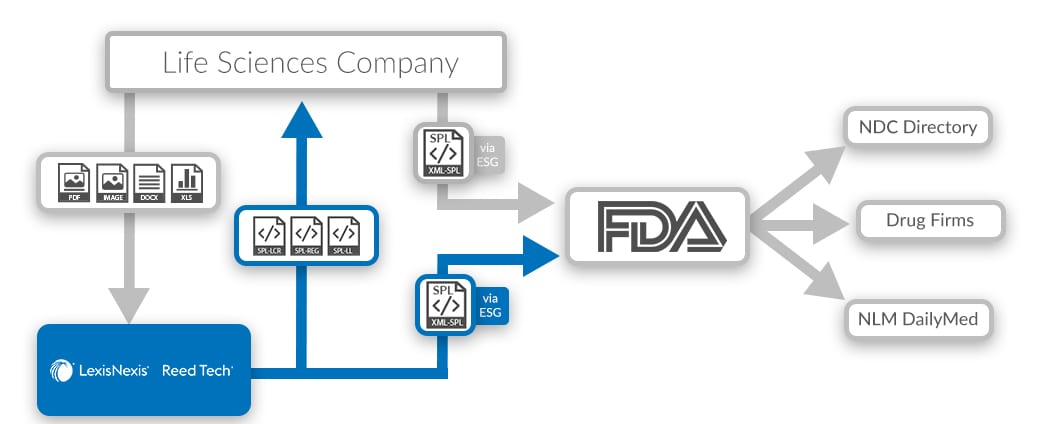
SPL for FDA Submission - Dakota Systems SPL is an HL7 standard that defines the content and structure of product labeling information required for submission to the FDA. It improves the integrity of product information through the use of consistent structure and standard terminology. It is used as the basis for regulatory guidance documents and applications for exchange of product labeling content.

Fda structured product labels
Structured Product Labeling (SPL) | Data Conversion Laboratory - DCL Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. NSDE | FDA - U.S. Food and Drug Administration CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA...
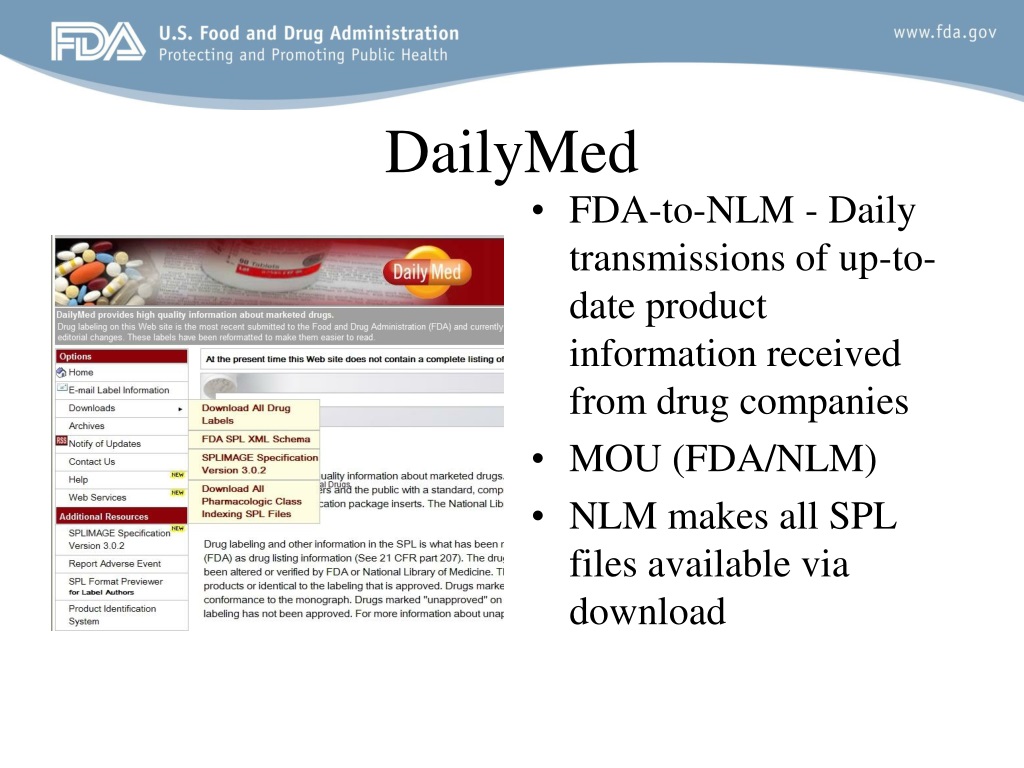
Fda structured product labels. FDALabel: Full-Text Search of Drug Product Labeling | FDA FDALabel Database is a web-based application that allows users to perform customizable searches of a ... FDA SPL for SPL R4 - Structured Product Labeling - User Manual The product label itself can be authored in Microsoft Word and then be loaded into the SPL document authored with FDA SPL application. Alternatively SPL document sections as well as coded information such as products, ingredients, product characteristics etc. can be imported from existing SPL files e.g. from those available on the Daily Med ... Structured Product Labeling Validation Rules - Food and Drug Administration Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ... FDA Label Search (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling on this...
SPL Xforms | FDA - U.S. Food and Drug Administration To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... NSDE | FDA - U.S. Food and Drug Administration CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA... Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. Structured Product Labeling (SPL) | Data Conversion Laboratory - DCL Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard.












![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f81b6e365340866e0eeb_Inner-Images-4.jpg)







Post a Comment for "39 fda structured product labels"